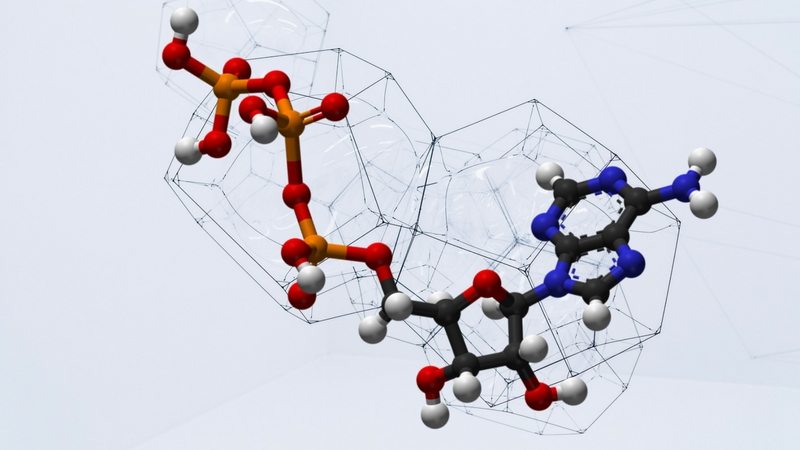
Nucleotides
Episode #2 of the course The molecular building blocks of life by Dr. Bill Thomas
In the previous lesson, we learned that carbohydrates, or sugars, are the building blocks for many larger molecules. In fact, a carbohydrate is one of three molecules needed to make a nucleotide, the molecular building block we’ll be looking at in today’s lesson.
A nucleotide is a three-part molecule. The first part is a carbohydrate such as we discussed in the previous lesson. One part of this carbohydrate is attached to a phosphate, a simple, small molecule consisting of a phosphorus atom surrounded by four oxygen atoms. Another part of the carbohydrate is attached to a base, which is a nitrogen-containing ring or double ring. The four bases found in DNA (adenine, cytosine, guanine, and thymine) are the same in every organism on earth! Since the sugar and phosphate molecules are the same from one nucleotide to the next, it is the base that differentiates these molecules from each other.
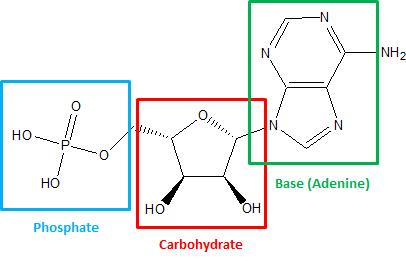
The three parts of a nucleotide
Nucleotides are present in every type of cell on Earth, from bacteria that inhabit volcanic fissures on the ocean floor to plants and animals (including humans!), and they serve a variety of functions that make life possible.
Nucleotide Functions
One of nucleotides’ important functions is to act as cellular messengers, carrying signals from one part of a cell to another. For instance, disease-causing bacteria may activate a receptor on the outside of a cell, stimulating it to send a warning to the interior of the cell to activate its internal defenses. Instead of radio waves or an audible cry for help, the receptor uses nucleotides to transmit the danger signal.
In addition to carrying signals throughout a cell, nucleotides are critically important as carriers of energy. Within every living cell is a frenzy of biological activity, with many thousands of biological processes being carried out at every moment. A great deal of energy is required to power all these processes, energy which the cell obtains from nucleotides.
Initially, all this energy comes from food (or photosynthesis, in the case of plants); however, the energy in food isn’t in a form that is readily available to most cellular processes. During metabolism, energy is released from the chemical bonds in food and stored in the chemical bonds of nucleotides, primarily a nucleotide called ATP. Because food energy is converted into this more usable form, ATP is often referred to as the “energy currency of the cell.”
ATP is readily broken down, releasing its stored energy to power many biological processes, including the muscle contractions that move your limbs or cause your heart to beat. In fact, every time you move, you are consuming ATP to release energy originally taken in as food.
Nucleotides are also the building blocks of the much larger biological molecule, DNA, which houses all of an organism’s genetic information. In fact, all this genetic information is spelled out using an “alphabet” of only four nucleotides.
Both DNA and its sister molecule, RNA, are made up of long chains of nucleotides, in which the phosphate of each nucleotide is chemically bound to the sugar of the nucleotide before it, and its sugar is chemically bound to the phosphate of the next nucleotide in the chain. This arrangement gives DNA and RNA molecules a phosphate-sugar “backbone,” with the bases of each nucleotide protruding inward from this backbone.
In the next lesson, we will take a closer look at how this simple arrangement of four nucleotides can be used to construct the extremely complex macromolecule, DNA, forming the repository of all genetic information.
Recommended book
The Double Helix: A Personal Account of the Discovery of the Structure of DNA by James Watson
Share with friends