Lipids
Episode #10 of the course The molecular building blocks of life by Dr. Bill Thomas
In previous lessons, we examined some of the molecular building blocks responsible for carrying out the biological activities that occur inside living cells. Now, we’ll take a look at lipids, a class of molecule responsible for containing the cellular environment in which all these biological processes occur.
What Is a Lipid?
While the terms “lipid,” “fat,” and “fatty acid” are often used interchangeably, lipids represent a diverse category of molecules that perform an amazing variety of functions within living cells. What these molecules have in common is that they all have at least one long hydrocarbon tail, formed by chains of carbon atoms that may exceed 20 carbons in length. Unlike carbohydrates, the carbon atoms in these chains are bound only to hydrogen (and to each other) and contain no oxygen atoms, giving lipids very different chemical properties from other molecules.
Fear of Water
The most important of these properties is that the hydrocarbon tails are hydrophobic, meaning they do not interact with water. Instead, the hydrocarbon tails of fats and other lipids associate tightly with each other; it is this property that prevents lipids such as fats, oils, and waxes from dissolving in water. If you have ever attempted to mix vegetable oil and water, you have probably seen the hydrophobic property of lipids in action.
Typically, multiple hydrocarbon tails are attached at one end by a molecule that is not hydrophobic; in the case of fatty acids, this molecule is glycerol, which has three hydrocarbon tails. Because living cells themselves are watery environments, lipids spontaneously arrange into membranes in which the hydrophobic tails associate with each other while presenting their non-hydrophobic “heads” to the watery environment inside (or outside) the cell. Without this critical membrane-forming property of lipids, cells could not exist.
Saturated versus Unsaturated Fats
Fats are said to be saturated when the carbon atoms in their hydrocarbon tails have formed the maximum number of possible bonds with hydrogen atoms (i.e., they are “saturated” with hydrogen atoms). If one or more of the carbon atoms in a tail form double bonds with each other instead of bonding to hydrogen, they are said to be unsaturated. These double bonds between carbon atoms introduce a sort of “kink” into the fatty acid tail that prevents it from associating as closely with its neighbors as a saturated tail. For this reason, unsaturated fats tend to be more fluid, which is why vegetable oils—which are mostly unsaturated—are liquid at room temperature, while animal fats—which are mostly saturated—are solid at room temperature.
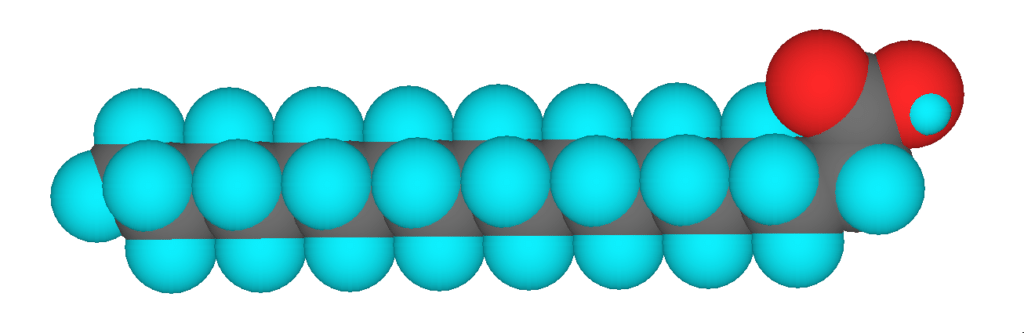
3D model of stearic acid, a saturated fatty acid
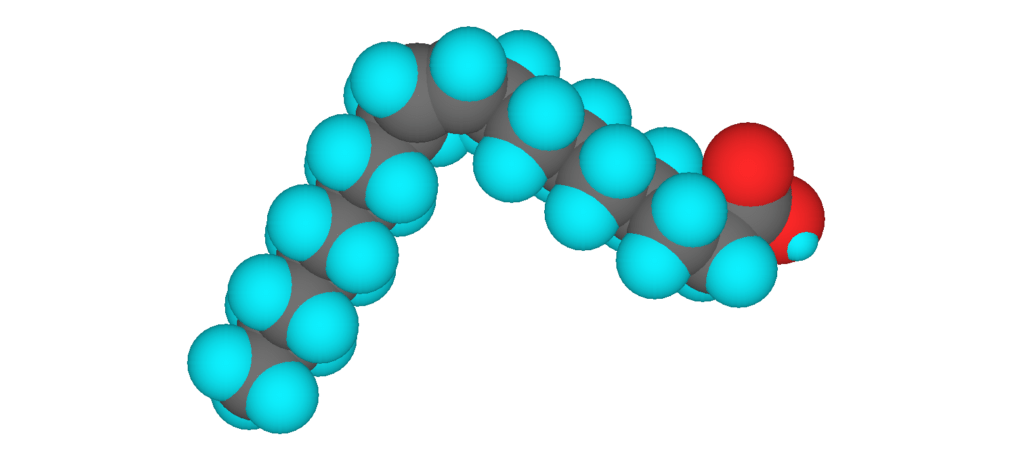
3D model of oleic acid, an unsaturated fatty acid
Steroids Are Lipids Too!
Another class of lipids, the sterols, is formed from hydrocarbons that have been arranged into a four-ring structure. This important class of lipids includes molecules such as cholesterol, which is a crucial component of cellular membranes, and signaling molecules such as the steroids testosterone and estrogen.
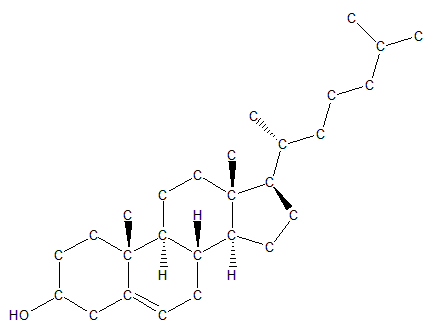
Cholesterol
Important Role of the Lipids
The remarkable membrane-forming property of lipids makes it possible for life as we know it to exist. The most basic unit of life—the cell—exists within a lipid membrane. Inside this membrane, all the biological processes necessary for life take place. In bacteria, this activity takes place within a single cellular membrane; however, in larger, more complex cells—including those of multicellular organisms like plants and animals—many smaller structures exist, all of which are defined by their own lipid membranes. These tiny internal organs of cells, called organelles, allow cells to perform a wide variety of specialized functions—and are quite impressive when viewed microscopically!
Congratulations on completing this course about the molecular building blocks of life! Hopefully, our tour of the molecules that make up our cells and carry out biological processes has impressed you with the marvelous feat of engineering that is the cell. The goal of this course was to help you get better acquainted with biological molecules that are frequently talked about but often poorly understood, and maybe introduce you to a few that you haven’t encountered before. The more you know about these incredible building blocks, the easier it is to appreciate nature’s most complex and impressive accomplishment: you!
Recommended book
The Vital Question: Energy, Evolution, and the Origins of Complex Life by Nick Lane
Share with friends

