Amino Acids
Episode #5 of the course The molecular building blocks of life by Dr. Bill Thomas
Previously, we looked at how the four nucleotides that make up DNA are used as a four-letter “alphabet” to generate the genetic code. Within a gene, the genetic code provides a blueprint that allows a cell to construct a protein out of amino acids. Now let’s take a deeper look at amino acids, the building blocks that make proteins possible.
The Building Blocks of Proteins
All the biological functions occurring in every living cell are carried out by proteins, tiny molecular machines that exist in millions of different forms, each built from a chain of amino acids. As mentioned in Lesson 4, there are 20 amino acids that form the basis of every protein. Unlike other molecular blocks, such as sugars, lipids, or nucleotides, which have relatively similar properties within each group, amino acids have distinctly different properties from each other. These differences make possible the wide diversity in protein structure and function.
Amino Acids Structure
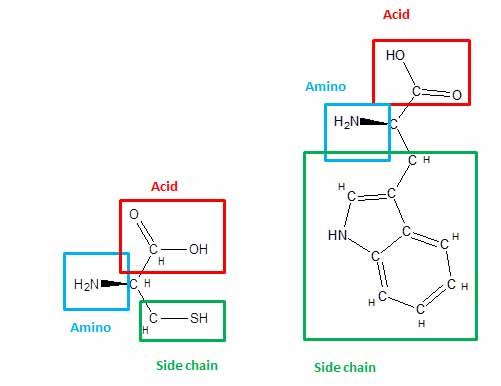
Like most of the molecular building blocks we’ve examined in this course, amino acids are made up of even smaller molecular building blocks, each with its own properties. Every amino acid consists of a carbon atom attached to a nitrogen group (the amino part of the molecule) and another carbon group (the acid). Individual amino acids are joined in long chains, nitrogen to carbon, via molecular bonds called peptide bonds. The completed chain of amino acids is called a polypeptide.
What Makes Them Different?
In addition to the amino group and the acid group, the central carbon is attached to a unique “side chain” that’s different for each amino acid. The side chain is a group of atoms that varies widely in composition and may include carbon, oxygen, nitrogen, or even sulfur. Some amino acids have very small side chains (glycine’s is just a single hydrogen atom), while others, such as tryptophan, have large, bulky ring structures. Some side chains carry a positive or a negative charge, while others are electrically neutral. The chemical properties of these side chains determine how individual amino acids interact with the watery environment within a cell and how they interact with each other.
Cysteine and Tryptophan
Chemical Properties of Amino Acids
The size and chemical properties of the side groups in a polypeptide chain influence the way they fit into the protein. The assembled amino acids attract and repel each other and are attracted and repelled by the watery environment of the cell, forcing the polypeptide to bend and fold into a final, three-dimensional protein shape.
It is important to note that the chemical properties of amino acids are affected by changes in pH (acidity) and temperature. When the environment of a cell becomes more (or less) acidic or in extremes of temperature, the amino acids in a protein interact differently with each other, bonds may become weakened, and the shape of a protein may become irreversibly altered, making it useless. This is why multicellular organisms must carefully regulate body temperature and pH. Humans, for instance, maintain a constant body temperature of 37°C (98.6°F); if you were to run a high temperature for an extended period of time, you might begin to suffer brain damage as your proteins were disrupted.
Essential Amino Acids
Interestingly, while plants are able to metabolically produce all 20 amino acids, animals can typically only make about half of them (humans can make eleven) and must get the rest of their amino acids from their diet. These “essential” amino acids are acquired by consuming protein, which is then broken down into its component amino acids through the process of digestion. These amino acids are then available for the consumer to use in building its own proteins. Thus, an organism deficient in one of its essential amino acids may have difficulty producing proteins needed for proper biological function.
Over the last few lessons, we’ve seen how nucleotides join together to form DNA, incorporated in a pattern called the genetic code. We’ve also discussed how that code provides a blueprint for cells, which allows them to build proteins out of amino acids. In the next lesson, we’ll take a deeper look at the amazing molecular machines called proteins and their importance in biology.
Recommended book
Molecular and Cell Biology for Dummies by René Fester Kratz
Share with friends

